- Strontium Noble Gas Configuration
- Strontium Valence Electron Configuration
- Strontium Valence Electrons Number
- Ground State Electron Configuration Strontium
“Having these two valence electrons means that there is more richness and complexity in the electronic states,” said Young. “It means that there are a broader range transitions.” Two Electrons—a Blessing and a Curse. Indeed, within the Strontium structure there are a plethora of optical transition widths. Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of strontium (atomic number: 38), the most. Oxidation States, 2. Electrons Per Shell, 2 8 18 8 2. Electron Configuration, Kr 5s 2. Valence electrons are those 'reactive' electrons found only in the Outer Energy shell of an atom. Strontium (Sr) has 2 valence electrons and Titanium (Ti) has 4. Strontium Electron configuration (5 Shells) is 2,8,18, 8 and 2 valence electrons). (Atomic number = 38) Titanium Electron configuration (4 Shells) is 2,8,10 and 2 (valence electrons).
- Electronegativity Chart
- Lead Electron Configuration
Electron Configuration For Strontium
Strontium Noble Gas Configuration
What is the Electron Configuration of Strontium?

Strontium Valence Electron Configuration
How Many Valence Electrons Does Strontium Have
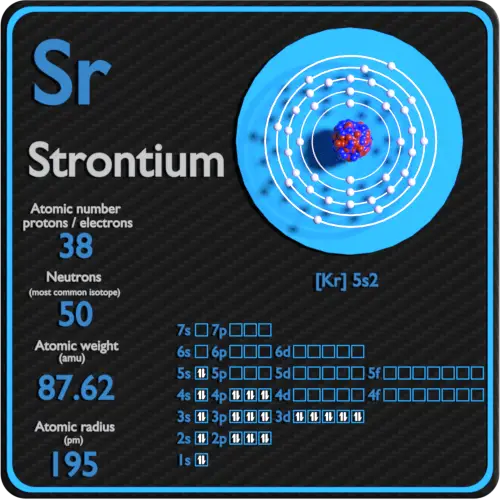
Strontium Number of Valence Electrons
Strontium Valence Electrons Number
Ground State Electron Configuration Strontium
